Introduction
Due to the rapidly evolving landscape of targeted therapies, there is an unmet need for comprehensive molecular profiling to guide treatment decisions for patients with lymphoma. To meet this need, we designed a pilot study to assess the feasibility and turnaround time (TAT) of comprehensive whole exome (WES) and whole transcriptome (RNA-seq) sequencing (Tumor Portrait test) in patients with lymphoma for future clinical decision-making (NCT05464823). Here, we present interim results for patients with large B-cell lymphoma (LBCL).
Methods
Patients ≥18 years of age with histologically documented LBCL requiring therapy were eligible. Formalin-fixed paraffin-embedded tissues underwent WES, RNA-seq, and copy number analysis with concomitant peripheral blood or saliva for germline DNA sequencing. During quality control, samples with <20% tumor purity and median coverage of <142x for tumor and/or <95x for normal samples were labeled as quantity not sufficient (QNS)-qualified. Genomic and transcriptomic data were profiled for alterations and molecular features to uncover clinically relevant biomarkers and match patients with clinical trials using data from ClinicalTrials.gov.
Results
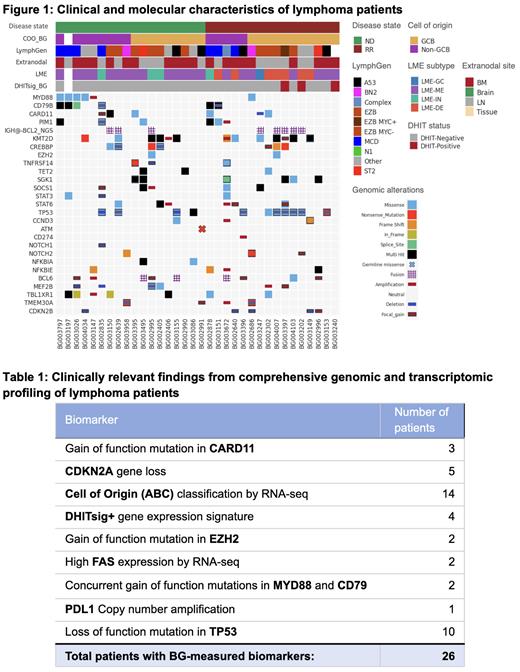
To date, 48 patients received WES and RNA-seq, and cohort data were analyzed for assay performance. Two samples were excluded from the analysis due to follicular lymphoma and chronic lymphocytic leukemia pathology, and seven samples were excluded as QNS. Reports were delivered for 39 patients, which included 37 clinical reports and two advanced reports lacking treatment and clinical trial information for QNS-qualified samples. While the QNS-qualified samples did not meet quality control standards, some data were still obtained and delivered as partial clinical reports. Of the 39 patients who received clinical reports, 34 samples were evaluable for bioinformatics analysis, and two of these samples had either only RNA-seq or WES data available (total n = 34, Figure 1). Cell of origin (COO), LymphGen (Wright et al. Cancer Cell. 2020), and lymphoma microenvironment (LME; Kotlov et al. Cancer Discov. 2021) classifications were applied to 33 patients. The median TAT was 8 days for advanced report delivery, and 85% of reports had a TAT <10 days.
WES identified frequent TP53 (n = 11) and KMT2D (n = 10) loss of function and MYD88 (n = 5) gain of function alterations, as anticipated for LBCL. COOs were assigned as activated (ABC; n = 14, 42%) or germinal center (GCB; n = 19, 58%) LBCL. Using the LymphGen classifier, EZB MYC- (n = 7, 22%), A53 (n = 6, 19%), and MCD (n = 5, 16%) were the most prevalent subtypes. LME classification revealed the majority of samples were mesenchymal (ME; n = 22, 67%). Immune-depleted (DE; n = 6, 18%), immune-inflamed (IN; n = 4, 12%), and germinal center-like (GC; n = 1, 3%) subtypes were less prevalent, but the immune-depleted LME occurred more commonly in patients at relapse. Clinically significant findings, such as DHITsig+ (n = 4) and CARD11 mutations indicative of ibrutinib resistance (n = 3), were identified in 26 of the 39 patients ( Table 1), and an average of 8 clinical trials were identified for potential patient enrollment in each delivered report.
Conclusions
These data indicated that comprehensive WES and RNA-seq molecular profiling can be performed for lymphoma patients with acceptable TAT. This approach identified significant alterations and classified LBCL samples into previously reported lymphoma subtypes. The clinical reports included clinically relevant findings and matched clinical trials, demonstrating the feasibility of incorporating comprehensive molecular profiling in clinical practice for patients with lymphoma.
Disclosures
Novokreshchenova:BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Bagaev:BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties. Kotlov:BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties. Postovalova:BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties. Shugaev-Mendosa:BostonGene, Corp.: Current Employment. Gracheva:BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Love:BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Nomie:BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Fowler:TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Flowers:Pfizer: Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Acerta: Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; Takeda: Research Funding; Adaptimmune: Research Funding; Bayer: Consultancy, Research Funding; Pharmacyclics: Research Funding; Celgene: Consultancy, Research Funding; Karyopharm: Consultancy; Jannsen Pharmaceuticals: Research Funding; Iovance: Research Funding; Sanofi: Research Funding; Burroghs Wellcome Fund: Research Funding; Nektar: Research Funding; Novartis: Research Funding; SeaGen: Consultancy; Genmab: Consultancy; Gilead: Consultancy, Research Funding; Pharmacyclics Jansen: Consultancy; Spectrum: Consultancy; 4D: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Kite: Research Funding; Morphosys: Research Funding; Genentech Roche: Consultancy, Research Funding; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Eastern Cooperative Oncology Group: Research Funding; V Foundation: Research Funding; National Cancer Institute: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Westin:Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Abbvie: Consultancy; SeaGen: Consultancy; Nurix: Consultancy; MonteRosa: Consultancy; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Calithera: Research Funding; Kymera: Research Funding; Kite/Gilead: Consultancy, Research Funding.